Sexually Transmitted Diseases Treatment Guidelines, 2010
Pregnant Women
Intrauterine or perinatally transmitted STDs can have severely debilitating effects on pregnant women, their partners, and their fetuses. All pregnant women and their sex partners should be asked about STDs, counseled about the possibility of perinatal infections, and provided access to treatment, if needed.
Recommended Screening Tests
-
All pregnant women in the United States should be screened for HIV infection as early in pregnancy as possible (77). Screening should be conducted after the woman is notified that she will be screened for HIV as part of the routine panel of prenatal tests, unless she declines (i.e., opt-out screening). For women who decline HIV testing, providers should address their objections, and when appropriate, continue to encourage testing strongly. Women who decline testing because they have had a previous negative HIV test should be informed of the importance of retesting during each pregnancy. Testing pregnant women and treating those who are infected are vital not only to maintain the health of the patient, but to reduce perinatal transmission of HIV through available antiretroviral and obstetrical interventions. Retesting in the third trimester (preferably before 36 weeks' gestation) is recommended for women at high risk for acquiring HIV infection (e.g., women who use illicit drugs, have STDs during pregnancy, have multiple sex partners during pregnancy, live in areas with high HIV prevalence, or have HIV-infected partners). Rapid HIV screening should be performed on any woman in labor who has an undocumented HIV status unless she declines. If a rapid HIV test result is positive in these women, antiretroviral prophylaxis should be administered without waiting for the results of the confirmatory test (78).
Anuncios -
A serologic test for syphilis should be performed on all pregnant women at the first prenatal visit (79). In populations in which the amount of prenatal care delivered is not optimal, rapid plasma reagin (RPR) card test screening (and treatment, if that test is reactive) should be performed at the time that a pregnancy is confirmed. Women who are at high risk for syphilis, live in areas of high syphilis morbidity, or are previously untested should be screened again early in the third trimester (at approximately 28 weeks' gestation) and at delivery. Some states require all women to be screened at delivery. Infants should not be discharged from the hospital unless the syphilis serologic status of the mother has been determined at least one time during pregnancy and preferably again at delivery. Any woman who delivers a stillborn infant should be tested for syphilis.
-
All pregnant women should be routinely tested for hepatitis B surface antigen (HBsAg) during an early prenatal visit (i.e., a visit during the first trimester), even if they have been previously vaccinated or tested (80). Women who were not screened prenatally, those who engage in behaviors that put them at high risk for infection (e.g., having had more than one sex partner in the previous 6 months, evaluation or treatment for an STD, recent or current injection-drug use, and an HBsAg-positive sex partner) and those with clinical hepatitis should be retested at the time of admission to the hospital for delivery. Pregnant women at risk for HBV infection also should be vaccinated. To avoid misinterpreting a transient positive HBsAg result during the 21 days after vaccination, HBsAg testing should be performed before vaccine administration. All laboratories that conduct HBsAg tests should use an FDA-cleared HBsAg test and perform testing according to the manufacturer's labeling, including testing of initially reactive specimens with a licensed neutralizing confirmatory test. When pregnant women are tested for HBsAg at the time of admission for delivery, shortened testing protocols can be used, and initially reactive results should prompt expedited administration of immunoprophylaxis to infants (80).
-
All pregnant women should be routinely screened for Chlamydia trachomatis (see Chlamydia Infections, Diagnostic Considerations) during the first prenatal visit (81). Women aged ≤25 years and those at increased risk for chlamydia (e.g., women who have a new or more than one sex partner) also should be retested during the third trimester to prevent maternal postnatal complications and chlamydial infection in the infant. Women found to have chlamydial infection during the first trimester should be retested within approximately 3--6 months, preferably in the third trimester. Screening during the first trimester might prevent the adverse effects of chlamydia during pregnancy, but supportive evidence for such screening is lacking.
-
All pregnant women at risk for gonorrhea or living in an area in which the prevalence of Neisseria gonorrhoeae is high should be screened at the first prenatal visit for N. gonorrhoeae (82). Women aged <25 years are at highest risk for gonorrhea infection. Other risk factors for gonorrhea include a previous gonorrhea infection, other STDs, new or multiple sex partners, inconsistent condom use, commercial sex work, and drug use. Pregnant women found to have gonococcal infection during the first trimester should be retested within approximately 3--6 months, preferably in the third trimester. Uninfected pregnant women who remain at high risk for gonococcal infection also should be retested during the third trimester.
-
All pregnant women at high risk for hepatitis C infection should be screened for hepatitis C antibodies (see Hepatitis C, Diagnostic Considerations) at the first prenatal visit. Women at high risk include those with a history of injection-drug use and those with a history of blood transfusion or organ transplantion before 1992.
-
Pregnant women should undergo a Papanicolau (Pap) test at the same frequency as nonpregnant women, although recommendations for their management differ (83,84).
Other Tests
-
- Evidence does not support routine testing for bacteria|l vaginosis (BV) in pregnancy. For asymptomatic pregnant women at high risk for preterm delivery, evidence is insufficient to assess the balance of benefits and harms of screening for BV (85). Symptomatic women should be evaluated and treated (see Bacterial Vaginosis).
Anuncios - Evidence does not support routine screening for Trichomonas vaginalis in asymptomatic pregnant women. Women who report symptoms should be evaluated and treated appropriately (see Trichomonas).
- Evidence does not support routine HSV-2 serologic screening among previously undiagnosed women during pregnancy.
Other Concerns
-
Pregnant women who are HBsAg positive should be reported to the local or state health department to ensure that they are entered into a case-management system and that timely and appropriate prophylaxis is provided for their infants. Information concerning the pregnant woman's HBsAg status should be provided to the hospital in which delivery is planned and to the health-care provider who will care for the newborn. In addition, household and sex contacts of women who are HBsAg positive should be vaccinated.
-
Women who are HBsAg positive should be provided with, or referred for, appropriate counseling and medical management. Pregnant women who are HBsAg positive should receive information regarding hepatitis B that addresses:
-- modes of transmission;
-- perinatal concerns (e.g., breastfeeding is not contraindicated);
-- prevention of HBV transmission, including the importance of postexposure prophylaxis for the newborn infant and hepatitis B vaccination for household contacts and sex partners; and
-- evaluation for and treatment of chronic HBV infection. -
No treatment is available for pregnant women infected with hepatitis C virus (HCV). However, all women with HCV infection should receive appropriate counseling and supportive care as needed (see Hepatitis C, Prevention). No vaccine is available to prevent HCV transmission.
-
In the absence of lesions during the third trimester, routine serial cultures for herpes simplex virus (HSV) are not indicated for women who have a history of recurrent genital herpes. Prophylactic cesarean delivery is not indicated for women who do not have active genital lesions at the time of delivery.
-
The presence of genital warts is not an indication for cesarean delivery.
For a more detailed discussion of STD testing and treatment among pregnant women, refer to the following references: Prenatal screening for HIV: A Review of the evidence for the U.S. Preventive Services Task Force (86); Revised Recommendations for HIV Testing of Adults, Adolescents, and Pregnant Women in Health-Care Setting (77); Guidelines for Perinatal Care (87); Rapid HIV Antibody Testing During Labor and Delivery for Women of Unknown HIV Status: A Practical Guide and Model Protocol (88); Viral Hepatitis in Pregnancy (89); Hepatitis B Virus: A Comprehensive Strategy for Eliminating Transmission in the United States --- Recommendations of the Immunization Practices Advisory Committee (ACIP) (4); Screening for Chlamydial Infection: U.S. Preventive Services Task Force Recommendation Statement (81); Canadian guidelines on sexually transmitted infections (90); USPSTF recommendations for STI screening (91); and Screening for Bacterial Vaginosis in Pregnancy to Prevent Preterm Delivery: U.S. Preventive Services Task Force Recommendation Statement (85).
Recommendations to screen pregnant women for STDs are based on disease severity and sequelae, prevalence in the population, costs, medicolegal considerations (e.g., state laws), and other factors. The screening recommendations in this report are generally broader (i.e., if followed, more women will be screened for more STDs than would by following other screening recommendations) and are also consistent with other CDC guidelines.
HIV Infection
All pregnant women in the United States should be tested for HIV infection as early during pregnancy as possible. A second test during the third trimester, preferably at <36 weeks' gestation, should be considered for all pregnant women and is recommended for women known to be at high risk for acquiring HIV, those who receive health care in jurisdictions with elevated incidence of HIV or AIDS among women, and women living in facilities in which prenatal screening identifies at least one HIV-infected pregnant women per 1,000 women screened (77). An RNA test should be used in conjunction with an HIV antibody test for women who have signs or symptoms consistent with acute HIV infection. The patient should first be informed that she will be tested for HIV as part of the panel of prenatal tests, unless she declines, or opts-out, of screening (77,86). For women who decline, providers should continue to strongly encourage testing and address concerns that pose obstacles to testing. Women who decline testing because they have had a previous negative HIV test should be informed about the importance of retesting during each pregnancy. Testing pregnant women is particularly important not only to maintain the health of the patient, but because interventions (i.e., antiretroviral and obstetrical) can reduce the risk for perinatal transmission of HIV.
After a pregnant woman has been identified as being HIV-infected, she should be educated about the risk for perinatal infection. Evidence indicates that, in the absence of antiretroviral and other interventions, 15%--25% of infants born to HIV-infected mothers will become infected with HIV; such evidence also indicates that an additional 12%--14% of infants born to infected mothers who breastfeed into the second year of life will become infected (138,139).
The risk for perinatal HIV transmission can be reduced to <2% through the use of antiretroviral regimens and obstetrical interventions (i.e., zidovudine or nevirapine and elective cesarean section at 38 weeks of pregnancy) and by avoiding breastfeeding (138,140). Pregnant women who are HIV-infected should be counseled concerning their options (either on-site or by referral), given appropriate antenatal treatment, and advised not to breastfeed their infants.
Diseases Characterized by Genital, Anal, or Perianal Ulcers
Chancroid
Successful treatment for chancroid cures the infection, resolves the clinical symptoms, and prevents transmission to others. In advanced cases, scarring can result, despite successful therapy.
|
Recommended Regimens |
|
Azithromycin 1 g orally in a single dose OR Ceftriaxone 250 mg intramuscularly (IM) in a single dose OR Ciprofloxacin* 500 mg orally twice a day for 3 days* OR Erythromycin base 500 mg orally three times a day for 7 days |
Ciprofloxacin is contraindicated for pregnant and lactating women. No adverse effects of chancroid on pregnancy outcome have been reported..
Azithromycin and ceftriaxone offer the advantage of single-dose therapy. Worldwide, several isolates with intermediate resistance to either ciprofloxacin or erythromycin have been reported. However, because cultures are not routinely performed, data are limited regarding the current prevalence of antimicrobial resistance.
Genital Herpes
Most mothers of infants who acquire neonatal herpes lack histories of clinically evident genital herpes (186). The risk for transmission to the neonate from an infected mother is high (30%--50%) among women who acquire genital herpes near the time of delivery and low (<1%) among women with histories of recurrent herpes at term or who acquire genital HSV during the first half of pregnancy (187). However, because recurrent genital herpes is much more common than initial HSV infection during pregnancy, the proportion of neonatal HSV infections acquired from mothers with recurrent herpes is substantial. Prevention of neonatal herpes depends both on preventing acquisition of genital HSV infection during late pregnancy and avoiding exposure of the infant to herpetic lesions during delivery. Because the risk for herpes is high in infants of women who acquire genital HSV during late pregnancy, these women should be managed in consultation with an infectious disease specialist.
Women without known genital herpes should be counseled to abstain from intercourse during the third trimester with partners known or suspected of having genital herpes. In addition, pregnant women without known orolabial herpes should be advised to abstain from receptive oral sex during the third trimester with partners known or suspected to have orolabial herpes. Some specialists believe that type-specific serologic tests are useful to identify pregnant women at risk for HSV infection and to guide counseling regarding the risk for acquiring genital herpes during pregnancy and that such testing should be offered to uninfected women whose sex partner has HSV infection. However, the effectiveness of antiviral therapy to decrease the risk for HSV transmission to pregnant women by infected partners has not been studied.
All pregnant women should be asked whether they have a history of genital herpes. At the onset of labor, all women should be questioned carefully about symptoms of genital herpes, including prodromal symptoms, and all women should be examined carefully for herpetic lesions. Women without symptoms or signs of genital herpes or its prodrome can deliver vaginally. Although cesarean section does not completely eliminate the risk for HSV transmission to the infant, women with recurrent genital herpetic lesions at the onset of labor should deliver by cesarean section to prevent neonatal HSV infection.
Treatment
First Clinical Episode of Genital Herpes
Newly acquired genital herpes can cause a prolonged clinical illness with severe genital ulcerations and neurologic involvement. Even persons with first-episode herpes who have mild clinical manifestations initially can develop severe or prolonged symptoms. Therefore, all patients with first episodes of genital herpes should receive antiviral therapy.
|
Recommended Regimens* |
|
Acyclovir 400 mg orally three times a day for 7--10 days OR Acyclovir 200 mg orally five times a day for 7--10 days OR Famciclovir 250 mg orally three times a day for 7--10 days OR Valacyclovir 1 g orally twice a day for 7--10 days |
|
*Treatment can be extended if healing is incomplete after 10 days of therapy. |
Episodic Therapy for Recurrent Genital Herpes
Effective episodic treatment of recurrent herpes requires initiation of therapy within 1 day of lesion onset or during the prodrome that precedes some outbreaks. The patient should be provided with a supply of drug or a prescription for the medication with instructions to initiate treatment immediately when symptoms begin.
|
Recommended Regimens |
|
Acyclovir 400 mg orally three times a day for 5 days OR Acyclovir 800 mg orally twice a day for 5 days OR Acyclovir 800 mg orally three times a day for 2 days OR Famciclovir 125 mg orally twice daily for 5 days OR Famciclovir 1000 mg orally twice daily for 1 day OR Famciclovir 500 mg once, followed by 250 mg twice daily for 2 days OR Valacyclovir 500 mg orally twice a day for 3 days OR Valacyclovir 1 g orally once a day for 5 days |
The safety of systemic acyclovir, valacyclovir, and famciclovir therapy in pregnant women has not been definitively established. Available data do not indicate an increased risk for major birth defects compared with the general population in women treated with acyclovir during the first trimester (188) --- findings that provide assurance to women who have had prenatal exposure to acyclovir. However, data regarding prenatal exposure to valacyclovir and famciclovir are too limited to provide useful information on pregnancy outcomes.
Acyclovir can be administered orally to pregnant women with first episode genital herpes or severe recurrent herpes and should be administered IV to pregnant women with severe HSV infection. Acyclovir treatment late in pregnancy reduces the frequency of cesarean sections among women who have recurrent genital herpes by diminishing the frequency of recurrences at term (189--191); the effect of antiviral therapy late in pregnancy on the incidence of neonatal herpes is not known. No data support the use of antiviral therapy among HSV seropositive women without a history of genital herpes.
Granuloma Inguinale (Donovanosis)
Several antimicrobial regimens have been effective, but only a limited number of controlled trials have been published (192). Treatment has been shown to halt progression of lesions, and healing typically proceeds inward from the ulcer margins; prolonged therapy is usually required to permit granulation and reepithelialization of the ulcers. Relapse can occur 6--18 months after apparently effective therapy.
Pregnancy is a relative contraindication to the use of sulfonamides. Pregnant and lactating women should be treated with the erythromycin regimen, and consideration should be given to the addition of a parenteral aminoglycoside (e.g., gentamicin). Azithromycin might prove useful for treating granuloma inguinale during pregnancy, but published data are lacking. Doxycycline and ciprofloxacin are contraindicated in pregnant women.
Lymphogranuloma Venereum
Treatment cures infection and prevents ongoing tissue damage, although tissue reaction to the infection can result in scarring. Buboes might require aspiration through intact skin or incision and drainage to prevent the formation of inguinal/femoral ulcerations.
Pregnant and lactating women should be treated with erythromycin. Azithromycin might prove useful for treatment of LGV in pregnancy, but no published data are available regarding its safety and efficacy. Doxycycline is contraindicated in pregnant women.
Syphilis
All women should be screened serologically for syphilis early in pregnancy. Most states mandate screening at the first prenatal visit for all women (231); antepartum screening by nontreponemal antibody testing is typical, but in some settings, treponemal antibody testing is being used. Pregnant women with reactive treponemal screening tests should have confirmatory testing with nontreponemal tests with titers. In populations in which use of prenatal care is not optimal, RPR test screening and treatment (if the RPR test is reactive) should be performed at the time that pregnancy is confirmed (232). For communities and populations in which the prevalence of syphilis is high and for patients at high risk, serologic testing should be performed twice during the third trimester (ideally at 28--32 weeks' gestation) and at delivery. Any woman who delivers a stillborn infant after 20 weeks' gestation should be tested for syphilis. No infant should leave the hospital without the maternal serologic status having been determined at least once during pregnancy.
Diagnostic Considerations
Seropositive pregnant women should be considered infected unless an adequate treatment history is documented clearly in the medical records and sequential serologic antibody titers have declined. Serofast low antibody titers might not require treatment; however, persistent higher titer antibody tests might indicate reinfection, and treatment might be required.
Treatment
Penicillin is effective for preventing maternal transmission to the fetus and for treating fetal infection (233). Evidence is insufficient to determine optimal, recommended penicillin regimens (234).
|
Recommended Regimen |
|
Pregnant women should be treated with the penicillin regimen appropriate for their stage of infection. |
Other Management Considerations
Some evidence suggests that additional therapy can be beneficial for pregnant women in some settings (e.g., a second dose of benzathine penicillin 2.4 million units IM administered 1 week after the initial dose for women who have primary, secondary, or early latent syphilis) (235). When syphilis is diagnosed during the second half of pregnancy, management should include a sonographic fetal evaluation for congenital syphilis, but this evaluation should not delay therapy. Sonographic signs of fetal or placental syphilis (i.e., hepatomegaly, ascites, hydrops, fetal anemia, or a thickened placenta) indicate a greater risk for fetal treatment failure (231); such cases should be managed in consultation with obstetric specialists. Evidence is insufficient to recommend specific regimens for these situations.
Women treated for syphilis during the second half of pregnancy are at risk for premature labor and/or fetal distress if the treatment precipitates the Jarisch-Herxheimer reaction (236). These women should be advised to seek obstetric attention after treatment if they notice any fever, contractions, or decrease in fetal movements. Stillbirth is a rare complication of treatment, but concern for this complication should not delay necessary treatment. All patients who have syphilis should be offered testing for HIV infection.
Follow-Up
Coordinated prenatal care and treatment are vital. Serologic titers should be repeated at 28--32 weeks' gestation and at delivery as recommended for the disease stage. Providers should ensure that the clinical and antibody responses are appropriate for the patient's stage of disease, although most women will deliver before their serologic response to treatment can be assessed definitively. Inadequate maternal treatment is likely if delivery occurs within 30 days of therapy, if clinical signs of infection are present at delivery, or if the maternal antibody titer at delivery is fourfold higher than the pretreatment titer. Serologic titers can be checked monthly in women at high risk for reinfection or in geographic areas in which the prevalence of syphilis is high
Penicillin Allergy
For treatment of syphilis during pregnancy, no proven alternatives to penicillin exist. Pregnant women who have a history of penicillin allergy should be desensitized and treated with penicillin. Oral step-wise penicillin dose challenge or skin testing might be helpful in identifying women at risk for acute allergic reactions (see Management of Patients Who Have a History of Penicillin Allergy).
Tetracycline and doxycycline usually are not used during pregnancy. Erythromycin and azithromycin should not be used, because neither reliably cures maternal infection or treats an infected fetus (234). Data are insufficient to recommend ceftriaxone for treatment of maternal infection and prevention of congenital syphilis.
HIV Infection
Placental inflammation from congenital infection might increase the risk for perinatal transmission of HIV. All HIV-infected women should be evaluated for syphilis and receive treatment as recommended. Data are insufficient to recommend a specific regimen for HIV-infected pregnant women (see Syphilis Among HIV-Infected Patients).
Congenital Syphilis
Effective prevention and detection of congenital syphilis depends on the identification of syphilis in pregnant women and, therefore, on the routine serologic screening of pregnant women during the first prenatal visit. In communities and populations in which the risk for congenital syphilis is high, serologic testing and a sexual history also should be obtained at 28 weeks' gestation and at delivery. Moreover, as part of the management of pregnant women who have syphilis, information concerning the treatment of sex partners should be obtained to assess the risk for reinfection.
Routine screening of newborn sera or umbilical cord blood is not recommended. Serologic testing of the mother's serum is preferred rather than testing of the infant's serum because the serologic tests performed on infant serum can be nonreactive if the mother's serologic test result is of low titer or the mother was infected late in pregnancy (see Diagnostic Considerations and Use of Serologic Tests). Screening can be performed using either a nontreponemal or treponemal test. If either screening test is positive, testing must be performed immediately using the other complimentary test (i.e., nontreponemal test followed by treponemal test or vice-versa). No infant or mother should leave the hospital unless maternal serologic status has been documented at least once during pregnancy; in communities and populations in which the risk for congenital syphilis is high, documentation should also occur at delivery.
Penicillin G, administered parenterally, is the preferred drug for treating all stages of syphilis. The preparation used (i.e., benzathine, aqueous procaine, or aqueous crystalline), the dosage, and the length of treatment depend on the stage and clinical manifestations of the disease. Selection of the appropriate penicillin preparation is important, because T. pallidum can reside in sequestered sites (e.g., the CNS and aqueous humor) that are poorly accessed by some forms of penicillin. Combinations of benzathine penicillin, procaine penicillin, and oral penicillin preparations are not considered appropriate for the treatment of syphilis. Reports have indicated that practitioners have inadvertently prescribed combination benzathine-procaine penicillin (Bicillin C-R) instead of the standard benzathine penicillin product (Bicillin L-A) widely used in the United States. Practitioners, pharmacists, and purchasing agents should be aware of the similar names of these two products to avoid using the inappropriate combination therapy agent for treating syphilis (206).
The effectiveness of penicillin for the treatment of syphilis was well established through clinical experience even before the value of randomized controlled clinical trials was recognized. Therefore, nearly all the recommendations for the treatment of syphilis are based not only on clinical trials and observational studies, but approximately 50 years of clinical experience.
Parenteral penicillin G is the only therapy with documented efficacy for syphilis during pregnancy. Pregnant women with syphilis in any stage who report penicillin allergy should be desensitized and treated with penicillin (see Management of Patients Who Have a History of Penicillin Allergy).
The Jarisch-Herxheimer reaction is an acute febrile reaction frequently accompanied by headache, myalgia, fever, and other symptoms that usually occur within the first 24 hours after the initiation of any therapy for syphilis. Patients should be informed about this possible adverse reaction. The Jarisch-Herxheimer reaction occurs most frequently among patients who have early syphilis, presumably because bacterial burdens are higher during these stages. Antipyretics can be used to manage symptoms, but they have not been proven to prevent this reaction. The Jarisch-Herxheimer reaction might induce early labor or cause fetal distress in pregnant women, but this should not prevent or delay therapy (see Syphilis During Pregnancy).
Primary and Secondary Syphilis
Parenteral penicillin G has been used effectively for more than 50 years to achieve clinical resolution (i.e., the healing of lesions and prevention of sexual transmission) and to prevent late sequelae. However, no comparative trials have been adequately conducted to guide the selection of an optimal penicillin regimen (i.e., the dose, duration, and preparation). Substantially fewer data are available for nonpenicillin regimens.
|
Recommended Regimen for Adults* |
|
Benzathine penicillin G 2.4 million units IM in a single dose |
|
* Recommendations for treating syphilis in HIV-infected persons and pregnant women are discussed later in this report (see Syphilis among HIV--Infected Persons and Syphilis in Pregnancy). |
Available data demonstrate that additional doses of benzathine penicillin G, amoxicillin, or other antibiotics in early syphilis (primary, secondary, and early latent) do not enhance efficacy, regardless of HIV status.
Latent Syphilis
Latent syphilis is defined as syphilis characterized by seroreactivity without other evidence of disease. Patients who have latent syphilis and who acquired syphilis during the preceding year are classified as having early latent syphilis. Patients' conditions can be diagnosed as early latent syphilis if, during the year preceding the evaluation, they had 1) a documented seroconversion or fourfold or greater increase in titer of a nontreponemal test; 2) unequivocal symptoms of primary or secondary syphilis; or 3) a sex partner documented to have primary, secondary, or early latent syphilis. In addition, for persons whose only possible exposure occurred during the previous 12 months, reactive nontreponemal and treponemal tests are indicative of early latent syphilis. In the absence of these conditions, an asymptomatic person should be considered to have late latent syphilis or syphilis of unknown duration. Nontreponemal serologic titers usually are higher during early latent syphilis than late latent syphilis. However, early latent syphilis cannot be reliably distinguished from late latent syphilis solely on the basis of nontreponemal titers. All patients with latent syphilis should have careful examination of all accessible mucosal surfaces (i.e., the oral cavity, perianal area, perineum and vagina in women, and underneath the foreskin in uncircumcised men) to evaluate for internal mucosal lesions. All patients who have syphilis should be tested for HIV infection.
Treatment
Because latent syphilis is not transmitted sexually, the objective of treating patients with this stage of disease is to prevent complications. Although clinical experience supports the effectiveness of penicillin in achieving this goal, limited evidence is available to guide choice of specific regimens.
The following regimens are recommended for penicillin nonallergic patients who have normal CSF examinations (if performed).
|
Recommended Regimens for Adults* |
|
Early Latent Syphilis Benzathine penicillin G 2.4 million units IM in a single dose |
|
Late Latent Syphilis or Latent Syphilis of Unknown Duration Benzathine penicillin G 7.2 million units total, administered as 3 doses of 2.4 million units IM each at 1-week intervals |
|
* Recommendations for treating syphilis in HIV-infected persons and pregnant women are discussed later in this report (see Syphilis among HIV-Infected Persons and Syphilis in Pregnancy). |
Available data demonstrate no enhanced efficacy of additional doses of penicillin G, amoxicillin, or other antibiotics in early syphilis, regardless of HIV status.
Tertiary Syphilis
Tertiary syphilis refers to gumma and cardiovascular syphilis but not to all neurosyphilis. Patients who are not allergic to penicillin and have no evidence of neurosyphilis should be treated with the following regimen.
|
Recommended Regimen |
|
Benzathine penicillin G 7.2 million units total, administered as 3 doses of 2.4 million units IM each at 1-week intervals |
Neurosyphilis
CNS involvement can occur during any stage of syphilis. However, CSF laboratory abnormalities are common in persons with early syphilis, even in the absence of clinical neurological findings. No evidence exists to support variation from recommended treatment for early syphilis for patients found to have such abnormalities. If clinical evidence of neurologic involvement is observed (e.g., cognitive dysfunction, motor or sensory deficits, ophthalmic or auditory symptoms, cranial nerve palsies, and symptoms or signs of meningitis), a CSF examination should be performed.
Syphilitic uveitis or other ocular manifestations frequently are associated with neurosyphilis and should be managed according to the treatment recommendations for neurosyphilis. Patients who have neurosyphilis or syphilitic eye disease (e.g., uveitis, neuroretinitis, and optic neuritis) should be treated with the recommended regimen for neurosyphilis; those with eye disease should be managed in collaboration with an ophthalmologist. A CSF examination should be performed for all patients with syphilitic eye disease to identify those with abnormalities; patients found to have abnormal CSF test results should be provided follow-up CSF examinations to assess treatment response.
|
Recommended Regimen |
|
Aqueous crystalline penicillin G 18--24 million units per day, administered as 3--4 million units IV every 4 hours or continuous infusion, for 10--14 days |
If compliance with therapy can be ensured, the following alternative regimen might be considered.
|
Alternative Regimen |
|
Procaine penicillin 2.4 million units IM once daily PLUS Probenecid 500 mg orally four times a day, both for 10--14 days |
The durations of the recommended and alternative regimens for neurosyphilis are shorter than the duration of the regimen used for late syphilis in the absence of neurosyphilis. Therefore, benzathine penicillin, 2.4 million units IM once per week for up to 3 weeks, can be considered after completion of these neurosyphilis treatment regimens to provide a comparable total duration of therapy.
No proven alternatives to penicillin are available for treating neurosyphilis, congenital syphilis, or syphilis in pregnant women. Pregnant patients who are allergic to penicillin should be desensitized and treated with penicillin.
Management of Persons Who Have a History of Penicillin Allergy
No proven alternatives to penicillin are available for treating neurosyphilis, congenital syphilis, or syphilis in pregnant women. Penicillin also is recommended for use, whenever possible, in HIV-infected patients. Of the adult U.S. population, 3%--10% have experienced an immunoglobulin E (IgE)-mediated allergic response to penicillin (238,239), such as urticaria, angioedema, or anaphylaxis (i.e., upper airway obstruction, bronchospasm, or hypotension). Readministration of penicillin to these patients can cause severe, immediate reactions. Because anaphylactic reactions to penicillin can be fatal, every effort should be made to avoid administering penicillin to penicillin-allergic patients, unless they undergo acute desensitization to eliminate anaphylactic sensitivity.
Although an estimated 10% of persons who report a history of severe allergic reactions to penicillin continue to remain allergic their entire lives, with the passage of time, most persons who have had a severe reaction to penicillin stop expressing penicillin-specific IgE (238,239). These persons can then be treated safely with penicillin. Penicillin skin testing with the major and minor determinants of penicillin can reliably identify persons at high risk for penicillin reactions (238,239). Although these reagents are easily generated and have been available for more than 30 years, only benzylpenicilloyl poly-L-lysine (Pre-Pen [i.e., the major determinant]) and penicillin G have been available commercially. These two tests identify an estimated 90%--97% of the currently allergic patients. However, because skin testing without the minor determinants would still miss 3%--10% of allergic patients and because serious or fatal reactions can occur among these minor-determinant--positive patients, caution should be exercised when the full battery of skin-test reagents is not available (Box 2). Manufacturers are working to ensure better availability of the Pre-Pen skin test reagent as well as an accompanying minor determinant mixture.
Recommendations
If the full battery of skin-test reagents is available, including both major and minor determinants (see Penicillin Allergy Skin Testing), patients who report a history of penicillin reaction and who are skin-test negative can receive conventional penicillin therapy. Skin-test--positive patients should be desensitized before initiating treatment.
If the full battery of skin-test reagents, including the minor determinants, is not available, the patient should be skin tested using benzylpenicilloyl poly-L-lysine (i.e., the major determinant) and penicillin G. Patients who have positive test results should be desensitized. One approach suggests that persons with a history of allergy who have negative test results should be regarded as possibly allergic and desensitized. Another approach in those with negative skin-test results involves test-dosing gradually with oral penicillin in a monitored setting in which treatment for anaphylactic reaction can be provided.
If the major determinant (Pre-Pen) is not available for skin testing, all patients with a history suggesting IgE-mediated reactions to penicillin (e.g., anaphylaxis, angioedema, bronchospasm, or urticaria) should be desensitized in a hospital setting. In patients with reactions not likely to be IgE-mediated, outpatient-monitored test doses can be considered.
Penicillin Allergy Skin Testing
Patients at high risk for anaphylaxis, including those who 1) have a history of penicillin-related anaphylaxis, asthma, or other diseases that would make anaphylaxis more dangerous or 2) are being treated with beta-adrenergic blocking agents, should be tested with 100-fold dilutions of the full-strength skin-test reagents before being tested with full-strength reagents. In these situations, patients should be tested in a monitored setting in which treatment for an anaphylactic reaction is available. If possible, the patient should not have taken antihistamines recently (e.g., chlorpheniramine maleate or fexafenadine during the preceding 24 hours, diphenhydramine HCl during the preceding 4 days, or hydroxyzine or phenathiazines during the preceding 3 weeks).
Procedures
Dilute the antigens either 100-fold for preliminary testing (if the patient has had a life-threatening reaction to penicillin) or 10-fold (if the patient has had another type of immediate, generalized reaction to penicillin within the preceding year).
Epicutaneous (Prick) Tests
Duplicate drops of skin-test reagent are placed on the volar surface of the forearm. The underlying epidermis is pierced with a 26-gauge needle without drawing blood. An epicutaneous test is positive if the average wheal diameter after 15 minutes is ≥4 mm larger than that of negative controls; otherwise, the test is negative. The histamine controls should be positive to ensure that results are not falsely negative because of the effect of antihistaminic drugs.
Intradermal Test
If epicutaneous tests are negative, duplicate 0.02-mL intradermal injections of negative control and antigen solutions are made into the volar surface of the forearm by using a 26- or 27-gauge needle on a syringe. The margins of the wheals induced by the injections should be marked with a ball point pen. An intradermal test is positive if the average wheal diameter 15 minutes after injection is >2 mm larger than the initial wheal size and also is >2 mm larger than the negative controls. Otherwise, the tests are negative.
Desensitization
Patients who have a positive skin test to one of the penicillin determinants can be desensitized (Table 1). This is a straightforward, relatively safe procedure that can be performed orally or IV. Although the two approaches have not been compared, oral desensitization is regarded as safer and easier to perform. Patients should be desensitized in a hospital setting because serious IgE-mediated allergic reactions can occur. Desensitization usually can be completed in approximately 4--12 hours, after which time the first dose of penicillin is administered. After desensitization, patients must be maintained on penicillin continuously for the duration of the course of therapy.
Chlamydial Infections
Doxycycline, ofloxacin, and levofloxacin are contraindicated in pregnant women. However, clinical experience and published studies suggest that azithromycin is safe and effective (289--291).
Repeat testing to document chlamydial eradication (preferably by NAAT) 3 weeks after completion of therapy with the following regimens is recommended for all pregnant women to ensure therapeutic cure, considering the severe sequelae that might occur in mothers and neonates if the infection persists. Women aged <25 years and those at increased risk for chlamydia (i.e., women who have a new or more than one sex partner) also should be retested during the third trimester to prevent maternal postnatal complications and chlamydial infection in the infant (81). Pregnant women diagnosed with a chlamydial infection during the first trimester should not only receive a test to document chlamydial eradication, but be retested 3 months after treatment.
|
Recommended Regimens |
|
Azithromycin 1 g orally in a single dose OR Amoxicillin 500 mg orally three times a day for 7 days |
|
Alternative Regimens |
|
Erythromycin base 500 mg orally four times a day for 7 days OR Erythromycin base 250 mg orally four times a day for 14 days OR Erythromycin ethylsuccinate 800 mg orally four times a day for 7 days OR Erythromycin ethylsuccinate 400 mg orally four times a day for 14 days |
The frequent gastrointestinal side effects associated with erythromycin can result in noncompliance with the alternative regimens. Although erythromycin estolate is contraindicated during pregnancy because of drug-related hepatotoxicity, the lower dose 14-day erythromycin regimens can be considered if gastrointestinal tolerance is a concern.
Follow-Up
Except in pregnant women, test-of-cure (i.e., repeat testing 3--4 weeks after completing therapy) is not advised for persons treated with the recommended or alterative regimens, unless therapeutic compliance is in question, symptoms persist, or reinfection is suspected. Moreover, the validity of chlamydial diagnostic testing at <3 weeks after completion of therapy (to identify patients who did not respond to therapy) has not been established. False-negative results might occur in the presence of persistent infections involving limited numbers of chlamydial organisms. In addition, NAAT conducted at <3 weeks after completion of therapy in persons who were treated successfully could yield false-positive results because of the continued presence of nonviable organisms (197).
A high prevalence of C. trachomatis infection has been observed in women and men who were treated for chlamydial infection during the preceding several months (251,267,286--288). Most post-treatment infections result from reinfection caused by failure of sex partners to receive treatment or the initiation of sexual activity with a new infected partner. Repeat infections confer an elevated risk for PID and other complications. Unlike the test-of-cure, which is not recommended, repeat C. trachomatis testing of recently infected women or men should be a priority for providers. Chlamydia-infected women and men should be retested approximately 3 months after treatment, regardless of whether they believe that their sex partners were treated (251,267). If retesting at 3 months is not possible, clinicians should retest whenever persons next present for medical care in the 12 months following initial treatment.
Gonococcal Infections
As with other patients, pregnant women infected with N. gonorrhoeae should be treated with a recommended or alternate cephalosporin. Because spectinomycin is not available in the United States, azithromycin 2 g orally can be considered for women who cannot tolerate a cephalosporin. Either azithromycin or amoxicillin is recommended for treatment of presumptive or diagnosed C. trachomatis infection during pregnancy.
Bacterial Vaginosis
BV is a polymicrobial clinical syndrome resulting from replacement of the normal hydrogen peroxide producing Lactobacillus sp. in the vagina with high concentrations of anaerobic bacteria (e.g., Prevotella sp. and Mobiluncus sp.), G. vaginalis, Ureaplasma, Mycoplasma, and numerous fastidious or uncultivated anaerobes. Some women experience transient vaginal microbial changes, whereas others experience them for a longer intervals of time. Among women presenting for care, BV is the most prevalent cause of vaginal discharge or malodor; however, in a nationally representative survey, most women with BV were asymptomatic (318).
Treatment is recommended for all pregnant women with symptoms. Although BV is associated with adverse pregnancy outcomes, including premature rupture of membranes, preterm labor, preterm birth, intra-amniotic infection, and postpartum endometritis, the only established benefit of therapy for BV in pregnant women is the reduction of symptoms and signs of vaginal infection. Additional potential benefits include reducing the risk for infectious complications associated with BV during pregnancy and reducing the risk for other infections (other STDs or HIV).
Several trials have been undertaken to determine the efficacy of BV treatment among pregnant women. Two trials demonstrated that metronidazole was efficacious during pregnancy using the 250-mg regimen (338,339); however, metronidazole administered at 500 mg twice daily can be used. One trial involving a limited number of participants revealed that treatment with oral metronidazole 500 mg twice daily was equally effective as metronidazole gel, with cure rates of 70% using Amsel criteria to define cure (340), and a recent trial demonstrated a cure rate of 85% using Gram stain criteria after 4 weeks with oral clindamycin (341). Multiple studies and meta-analyses have not demonstrated an association between metronidazole use during pregnancy and teratogenic or mutagenic effects in newborns (342,343). Regardless of the antimicrobial agent used to treat pregnant women, oral therapy is preferred because of the possibility of subclinical upper-genital--tract infection.
|
Recommended Regimens for Pregnant Women |
|
Metronidazole 500 mg orally twice a day for 7 days OR Metronidazole 250 mg orally three times a day for 7 days OR Clindamycin 300 mg orally twice a day for 7 days |
Treatment of asymptomatic BV among pregnant women who are at high risk for preterm delivery (i.e., those with a previous preterm birth) has been evaluated by several studies, which have yielded mixed results. Seven trials have evaluated treatment of pregnant women with asymptomatic BV at high risk for preterm delivery; one showed harm (344), two showed no benefit (345,346), and four demonstrated benefit (338,339,347,348). Therefore, evidence is insufficient to assess the impact of screening for BV in pregnant women at high risk for preterm delivery (85).
Similarly, data are inconsistent regarding whether the treatment of asymptomatic pregnant women with BV who are at low risk for preterm delivery reduces adverse outcomes of pregnancy. Although USPSTF recommends against screening these women (85), one trial demonstrated a 40% reduction in spontaneous preterm birth among women using oral clindamycin during weeks 13--22 of gestation (348). Several additional trials have shown that intravaginal clindamycin given at an average gestation of later than 20 weeks did not reduce preterm birth, and in three of these trials, intravaginal clindamycin cream administered at 16--32 weeks' gestation was associated with an increase in adverse events (e.g., low birth weight and neonatal infections) in newborns (346,349--351). Providers should be aware that intravaginal clindamycin cream might be associated with adverse outcomes if used in the latter half of pregnancy.
Trichomoniasis
Vaginal trichomoniasis has been associated with adverse pregnancy outcomes, particularly premature rupture of membranes, preterm delivery, and low birth weight. However, metronidazole treatment has not been shown to reduce perinatal morbidity. Although some trials suggest the possibility of increased prematurity or low birth weight after metronidazole treatment, limitations of the studies prevent definitive conclusions regarding risks for treatment (367,368). Treatment of T. vaginalis might relieve symptoms of vaginal discharge in pregnant women and might prevent respiratory or genital infection of the newborn and further sexual transmission. Clinicians should counsel patients regarding the potential risks and benefits of treatment and communicate the option of therapy deferral in asymptomatic pregnant women until after 37 weeks' gestation. All symptomatic pregnant women should not only be considered for treatment regardless of pregnancy stage, but be provided careful counseling regarding condom use and the continued risk of sexual transmission.
Women can be treated with 2 g metronidazole in a single dose at any stage of pregnancy. Multiple studies and meta-analyses have not demonstrated an association between metronidazole use during pregnancy and teratogenic or mutagenic effects in infants (342,343,369). The safety of tinidazole in pregnant women, however, has not been well evaluated.
In lactating women who are administered metronidazole, withholding breastfeeding during treatment and for 12--24 hours after the last dose will reduce the exposure of the infant to metronidazole. For women treated with tinidazole, interruption of breastfeeding is recommended during treatment and for 3 days after the last dose.
Vulvovaginal Candidiasis
Vulvovaginal Candidiasis frequently occurs during pregnancy. Only topical azole therapies, applied for 7 days, are recommended for use among pregnant women.
Pelvic Inflammatory Disease
Because of the high risk for maternal morbidity and preterm delivery, pregnant women who have suspected PID should be hospitalized and treated with parenteral antibiotics.
Human Papillomavirus (HPV) Infection
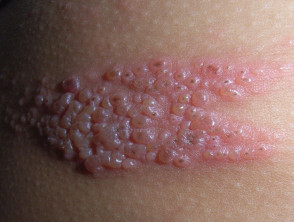
Imiquimod, sinecatechins, podophyllin, and podofilox should not be used during pregnancy. Genital warts can proliferate and become friable during pregnancy. Although removal of warts during pregnancy can be considered, resolution might be incomplete or poor until pregnancy is complete. Rarely, HPV types 6 and 11 can cause respiratory papillomatosis in infants and children, although the route of transmission (i.e., transplacental, perinatal, or postnatal) is not completely understood. Whether cesarean section prevents respiratory papillomatosis in infants and children also is unclear (412); therefore, cesarean delivery should not be performed solely to prevent transmission of HPV infection to the newborn. Cesarean delivery is indicated for women with genital warts if the pelvic outlet is obstructed or if vaginal delivery would result in excessive bleeding. Pregnant women with genital warts should be counseled concerning the low risk for warts on the larynx (recurrent respiratory papillomatosis) in their infants or children.
Hepatitis B
CDC's national strategy to eliminate transmission of HBV infection includes 1) prevention of perinatal infection through routine screening of all pregnant women for HBsAg and immunoprophylaxis of infants born to HBsAg-positive mothers or mothers whose HBsAg status is unknown, 2) routine infant vaccination, 3) vaccination of previously unvaccinated children and adolescents through age 18 years, and 4) vaccination of previously unvaccinated adults at increased risk for infection (3,4). High vaccination coverage rates, with subsequent declines in acute hepatitis B incidence, have been achieved among infants and adolescents (4,437,443). In contrast, vaccination coverage among most high-risk adult groups (e.g., persons with more than one sex partner in the previous 6 months, MSM, and IDUs) has remained low, and most new infections occur in these high-risk groups (3,108,444--446). STD clinics and other settings that provide services to high-risk adults are ideal sites in which to provide hepatitis B vaccination to adults at risk for HBV infection. All unvaccinated adults seeking services in these settings should be assumed to be at risk for hepatitis B and should be offered hepatitis B vaccination.
All pregnant women receiving STD services should be tested for HBsAg, regardless of whether they have been previously tested or vaccinated. All HBsAg-positive pregnant women should be reported to state and local perinatal hepatitis B prevention programs. HBsAg-negative pregnant women seeking STD treatment who have not been previously vaccinated should receive hepatitis B vaccination. Additional information regarding management of HBsAg-positive pregnant women and their infants is available at http://www.cdc.gov/mmwr/PDF/rr/rr5416.pdf ![]() .
.
No specific therapy is available for persons with acute hepatitis B; treatment is supportive. Persons with chronic HBV infection should be referred for evaluation to a physician experienced in the management of CLD. Therapeutic agents cleared by FDA for treatment of chronic hepatitis B can achieve sustained suppression of HBV replication and remission of liver disease in some persons. In addition, patients with chronic hepatitis B might benefit from screening to detect HCC at an early stage.
Hepatitis C
Routine testing for HCV infection is not recommended for all pregnant women. Pregnant women with a known risk factor for HCV infection should be offered counseling and testing. Patients should be advised that approximately six of every 100 infants born to HCV-infected woman become infected; this infection occurs predominantly during or near delivery, and no treatment or delivery method---such as caesarian section---has been demonstrated to decrease this risk. The risk is increased, however, by the presence of maternal HCV viremia at delivery and also is greater (2--3 times) if the woman is coinfected with HIV. HCV has not been shown to be transmitted through breast milk, although HCV-positive mothers should consider abstaining from breastfeeding if their nipples are cracked or bleeding. Infants born to HCV-positive mothers should be tested for HCV infection and, if positive, evaluated for the presence of CLD.
Ectoparasitic Infections
Pediculosis Pubis
Pregnant and lactating women should be treated with either permethrin or pyrethrins with piperonyl butoxide; lindane and ivermectin are contraindicated in pregnancy and lactating women.
Scabies
Infants, young children, and pregnant or lactating women should not be treated with lindane; however, they can be treated with permethrin. Ivermectin is not recommended for pregnant or lactating patients, and the safety of iver